What is Zink?
What Is Zinc
Scientific Data
- Chemical Symbol Zn
- Atomic Number 30
- Chemical Series Transition metals
- Density 7140kg/m3
- Appearance Bluish pale grey
- Melting Point 420°C
- Boiling Point 907°C
- Heat of Vaporisation 115.3 kJ/mol
- Heat of Fusion 7.3 kJ/mol
- Specific Heat Capacity 390 J/(kg.K)
- Electrical conductivity 16.6 106(m.ohm)
- Thermal conductivity 116 W(m.K)
Notable Characteristics
Zinc is a moderately reactive metal that will combine with oxygen and other non-metals and will react with dilute acids to release hydrogen.
History
The earliest use of zinc was in brass where it is alloyed with copper. This use probably arose accidentally when zinc-containing raw materials were reduced with charcoal in a copper crucible. These developments cannot be precisely dated but were well developed by 20 BC when the Romans were using brass in coinage. Experimental observations in Greece and Babylon predate this widespread use by at least two centuries. It is likely that the bronzes that lent their name to the archaeological age of 3000 BC – 1000 BC contained some zinc by accident or design. Brass was also known in India and China early in their recorded histories.
Occurrence
The world is naturally abundant in zinc. It is estimated that the first mile of the earth’s crust under land contains 224,000,000 million tonnes of zinc. Such estimate, however, take no account of whether or not it is economic or environmentally acceptable to exploit these resources.
The most common zinc mineral is sphalerite also known as zinc blende. This mineral crystallises from the hydrothermal solution as pure zinc sulphide and is found in almost all currently mined zinc deposits. Zinc is often mined in association with lead, copper, silver and other metals.
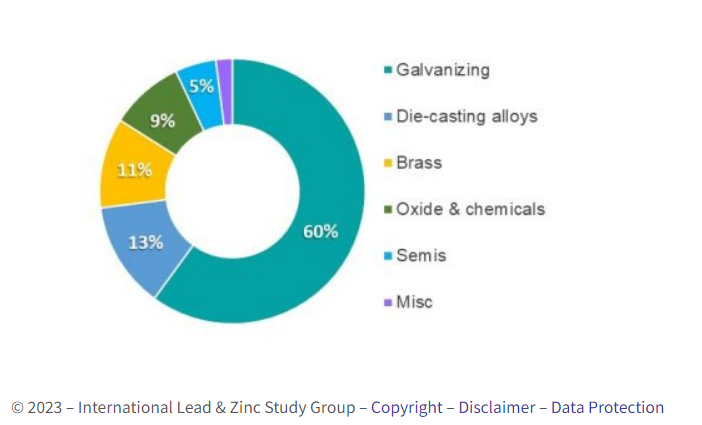
End Uses of Zinc
Zinc’s effectiveness in protecting steel against corrosion by galvanising is well recognised, while its ability to die cast complicated components makes zinc indispensable in a multitude of industry and household products. It also has important markets in the brass and construction industries and in chemicals and constitutes an essential nutritional element. Zinc’s main end uses are shown in the chart below.
source/ILZSG