What is Lead?
What Is Lead
Scientific Data
- Chemical Symbol Pb
- Atomic Number 82
- Chemical Series Poor metals
- Density 11340 kg/m3
- Appearance Bluish white
- Melting Point 327°C
- Boiling Point 1749°C
- Heat of Vaporisation 177.7 kJ/mol
- Heat of Fusion 4.8 kJ/mol
- Specific Heat Capacity 129 J/(kg.K)
- Electrical conductivity 4.81 MS/m
- Thermal conductivity 35.3W/(m.K)
Notable Characteristics
Lead has a bright lustre and is a dense, ductile, very soft, highly malleable, bluish-white metal that has poor electrical conductivity. It is also highly resistant to corrosion and because of this property is used to contain corrosive liquids (e.g. sulphuric acid).
History
Humans have used lead for at least 7,000 years mainly because deposits containing lead are widespread and it is easy to extract and work with. Lead was mentioned in the book of Exodus. Alchemists thought that lead was the oldest metal and associated it with the planet Saturn. Lead pipes bearing the insignia of Roman emperors are symbol in service today in some countries. Lead’s symbol Pb is an abbreviation of its Latin name plumbum. The English word plumbing also derives from this Latin root.
Occurrence
Lead is usually found in ore with zinc, silver and copper and is extracted together with these metals. The main lead mineral is galena (PbS). Other common varieties include cerussite (PbCO3) and angelsite (PbSO4).
End Uses of Lead
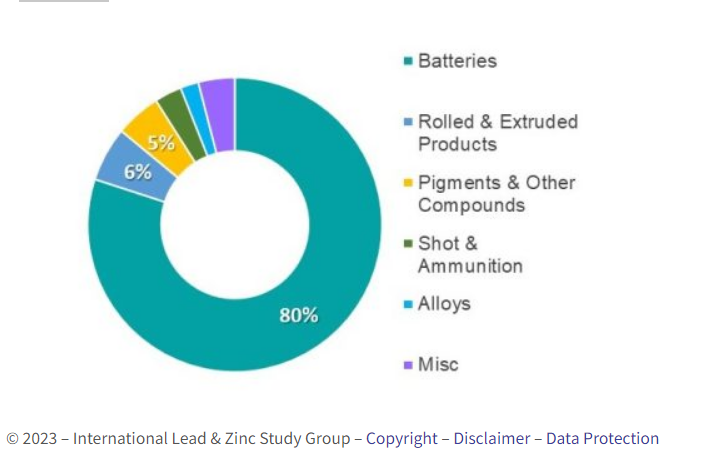
The principal use of lead is for lead-acid batteries for vehicles, backup emergency systems, the telecoms sector and fork lift trucks. Lead is also used in rolled and extruded products, compounds in the glass and plastics industries, shot and ammunition and for radiation shielding. The end use breakdown for lead is illustrated in the chart below.
source/ILZSG